BLOG
What is critical load in dental implants?
In dental implantology, one of the most relevant concepts from the biomechanical point of view is the critical load. This
Differences between MDR and MDD for manufacturers
The move from Directive 93/42/EEC (MDD) to Regulation (EU) 2017/745 (MDR) has brought about one of the biggest regulatory changes
What tests must a dental implant pass before it reaches the market?
The development of a dental implantable medical device does not end when the design is finalized. Before reaching the market,
Why should osteosynthesis plates be validated according to ASTM F382?
Osteosynthesis plates are implantable medical devices designed to stabilize bone fractures and allow proper bone healing. During the healing process,
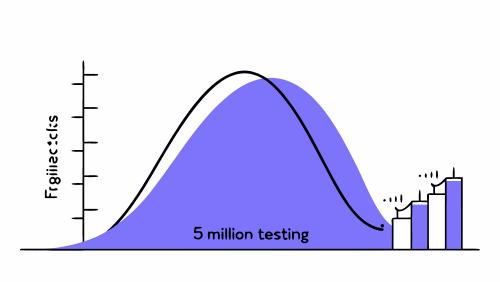
Fatigue testing: what do 5 million cycles mean in clinical practice?
In the development of an implantable medical device, few figures appear as often in technical reports as 5 million cycles.
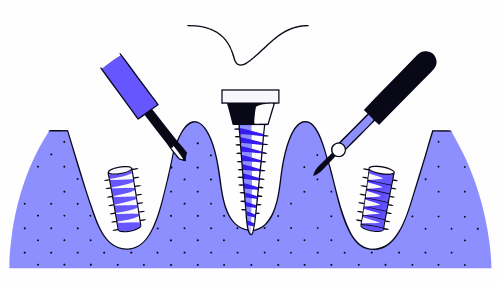
How osseointegration is measured in dental and orthopedic implants
Osseointegration is one of the determining factors for the long-term success of an implantable medical device. In both dental implantology

