MECHANICAL TESTING LABORATORY
The evaluation of medical devices requires precision, experience, and in-depth
knowledge of current regulations. At the At the IBV’s Mechanical Testing Laboratory, we perform specialized tests to ensure the safety, strength and durability of product before commercialization.
Our tests focus on the mechanical evaluation of implants, prostheses and orthoses,applying benchmark methods to meet the most demanding standards. In addition to provide technical results, we offer differential added value through data interpretation and advice on product design optimization.
MECHANICAL TESTS FOR MEDICAL DEVICES
At the IBV, we have extensive experience conducting mechanical testing on medical devices, covering everything from structural strength tests to load and fatigue simulations, among many others.
In this way, we evaluate the compliance of products such as dentalimplants, spine implants, hip implants, knee implants, bone plates,finger joint implants, assistive devices, exoskeletons, among many others.
Our laboratory adapts its tests to innovative projects, developing its own specific protocols when no applicable regulations exist.
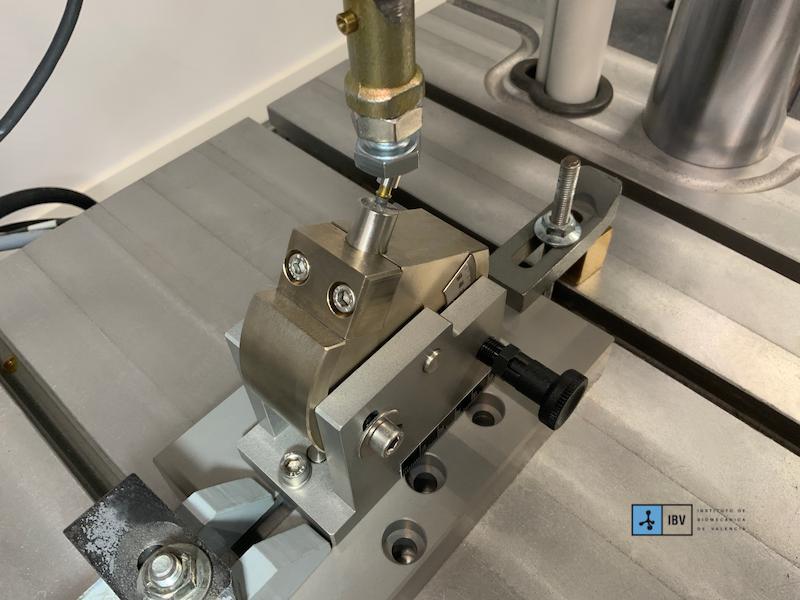
TECNOLOGÍA Y EQUIPAMIENTO AVANZADO
To ensure precise and reproducible results, we use state-of-the-art technology.
This infrastructure allows us to replicate real-world usage conditions and deliver critical data for the improvement of medical device design and manufacturing.

REGULATORY COMPLIANCE AND CE MARKING SUPPORT
Compliance with regulations is a key factor in the evaluation of medical products and devices. In our laboratory, we carry out tests in accordance with international standards. We also support manufacturers and companies in obtaining CE marking, based on the European Medical Device Regulation (MDR) 2017/745.
WHY CHOOSE IBV’S MECHANICAL TESTING LABORATORY?
If you’re looking for a mechanical testing laboratory to evaluate your products’ compliance with applicable regulations, contact our team of experts.
- Experience and accreditations: A benchmark in medical product evaluation, with recognized accreditations.
- Cutting-edge technology: Advanced equipment for both standard and custom mechanical testing.
- Results interpretation and guidance: Beyond raw data, we offer technical support to help companies in product optimization processes.
- Support in generating and drafting technical documentation for CE marking: We help companies compile the full technical file required for CE certification of their medical devices.
- Support in the preparation and drafting of technical documentation for CE marking: We assist companies in compiling the technical file required for the CE marking of their medical devices.
Contact our technical team
We’ll help you define the testing required to assess the compliance of your medical products for market access.

