TESTING OF
OSTEOSYNTHESIS IMPLANTS
The Institute of Biomechanics (IBV) is a reference center in the testing of bone plates, screws, pins, nails and external fixators, offering specializedThe IBV offers specialized tests to analyze the mechanical behavior of these devices before their certification or commercialization.
At IBV we perform advanced mechanical tests on materials and structures to evaluate their resistance to fatigue, bending, torsion and static loading, providing key data for the optimization of the design and selection of materials in osteosynthesis systems. These studies are essential for manufacturers seeking to validate new configurations, improve product safety or explore innovations in biomaterials..
In addition to technical results, we offer strategic recommendations to optimize the resistance, durability and functionality of the devices, reducing risks in their clinical application.
TESTING OF OSTEOSYNTHESIS PLATES
The osteosynthesis plates are key devices in orthopedic and maxillofacial surgery to stabilize fractures and facilitate bone regeneration. At IBV we carry out trials that evaluate their structural strength, durability and mechanical behavior under different loading conditions..
- Four-point bending tests (ISO 9585 / ASTM F382)Evaluation of structural strength and stiffness of bone plates.
- Fatigue testing of metallic platesDetermination of the durability of the material under prolonged load cycles.
- Impact and fracture toughness testingSimulation of sudden loads to analyze structural integrity.
- Structural validation according to ASTM F384: Evaluation of orthopedic metal devices subjected to angular loads.
ASTM F3437-23: Evaluation of metallic plates in small fractures.

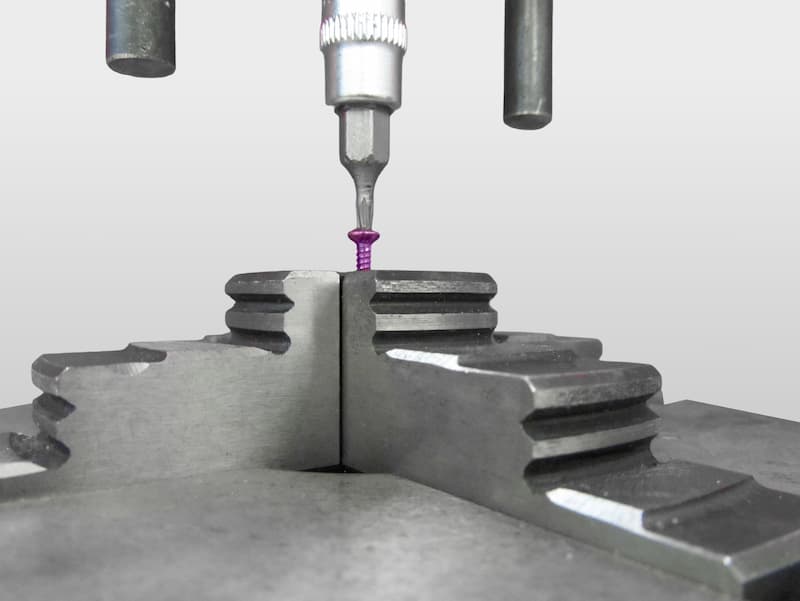
SCREW TESTS
Bone screws are essential in plate fixation and independent osteosynthesis procedures. The strength and stability of these devices are crucial to ensure their long-term functionality.
- Axial load tests on bolts (ASTM F543): Measurement of fastening capacity and pull-out resistance.
- Torsion testing of orthopedic screws: Evaluation of rotational strength and fixation stability.
- Fatigue testing of osteosynthesis screws: Analysis of behavior under repetitive loading in simulated clinical conditions.
TRIALS ON INTRAMEDULLARY NAILS
Intramedullary nails are used in fractures of long bones such as the femur and tibia. Their validation requires specific tests that evaluate their stability and durability in different types of fractures.
- Load tests on intramedullary nails (ASTM F1264): Flexure and fatigue tests on intramedullary osteosynthesis devices.
- Compressive and shear strength testing: Evaluation of nail response to vertical and lateral loading forces.
- Fatigue test on locking screws: Determination of the durability of fixation points on the intramedullary nail.

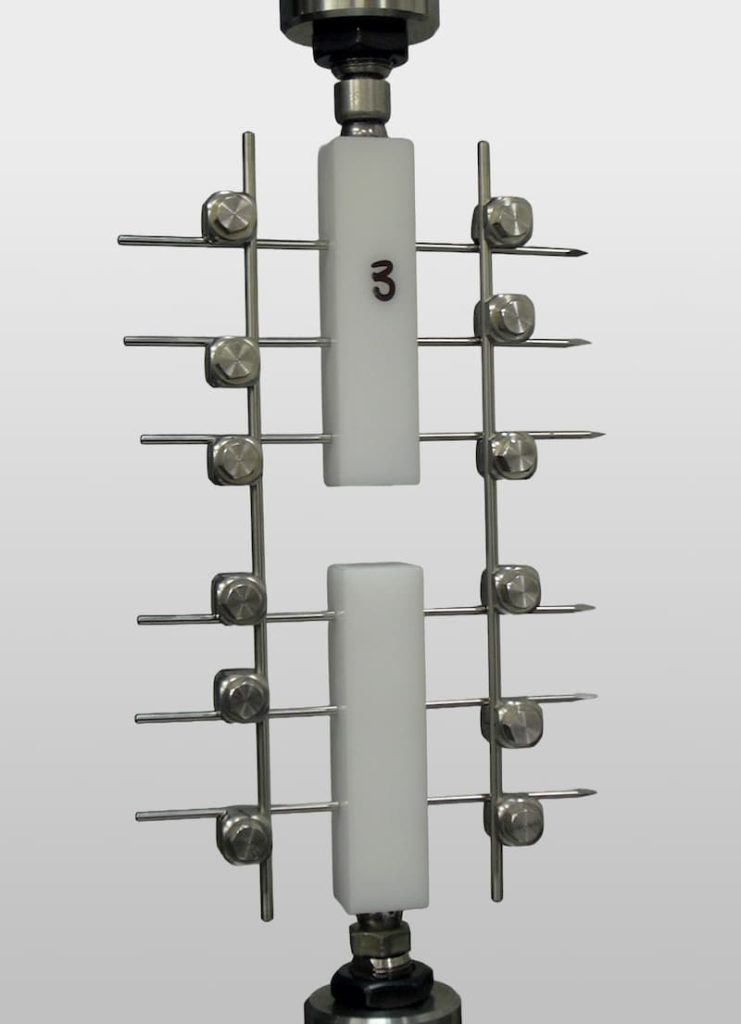
TESTS ON EXTERNAL FIXATORS
External fixators are adjustable systems used in open fractures and reconstructive surgeries. Their evaluation should focus on the strength of the structure and stability under variable loading conditions.
- Evaluation of mechanical behavior in external fixators (ASTM F1541): Analysis of assembly stability in different clinical scenarios.
- Stiffness and structural stability tests: Measurement of the mechanical response to multidirectional loads.
- Strength testing of fasteners: Evaluation of the integrity of the connections between the various components of the external fixator.
TRIALS ON BONE STAPLES
Bone staples are used in the fixation of minor fractures and in bone reconstruction procedures. Their fixation stability and fatigue resistance are key aspects in their validation.
- Flexural strength tests on bone staples (ASTM F564): Tests to determine their ability to withstand repetitive bending loads.
- Bone tissue fixation tests: Evaluation of the force required for staple insertion and removal.
- Fatigue testing of surgical staples: Simulation of prolonged use under mechanical loading.


EVALUATION OF MATERIALS IN OSTEOSYNTHESIS DEVICES
The performance of an osteosynthesis system depends on both the structural design and the properties of the material used. At IBV we perform specific tests to analyze the mechanical behavior of different biomaterials.
- Tensile and compression testing: Strength evaluation of titanium alloys, stainless steel and biomedical polymers.
- Coating adhesion tests (ASTM F1147): Determination of the stability of ceramic and metallic coatings on plates and screws.
- Wear and friction tests: Analysis of surface deterioration in devices subjected to continuous contact with bone or soft tissues.
- Structural stiffness evaluation: analysis of the modulus of elasticity in osteosynthesis devices to guarantee their behavior under load.
WHY CHOOSE IBV FOR OSTEOSYNTHESIS ASSAYS
The evaluation of osteosynthesis systems requires a comprehensive comprehensive analysis combining mechanical testing, materials evaluation and clinical environment interaction testing..
At IBV we offer:
- Mechanical testing laboratoryFatigue, torsion and resistance tests on osteosynthesis devices.
- Equipment specialized in biomaterials and orthopedic biomechanics..
- Methodology adapted to each manufacturerCustomized tests according to the design and materials used.
- Strategic analysisWe provide technical recommendations to improve the safety and efficiency of devices prior to certification.
Contact our technical team
Find out how our tests can help you evaluate and improve the performance of your osteosynthesis systems.

