The four-point bending test is one of the most widely used mechanical tests to validate the strength and stiffness of structural components. In the field of medical devices, this test is particularly relevant for evaluating products subjected to repetitive loads and bending stresses, such as osteosynthesis platesinternal fixations, implantable metal components and certain surgical instrumentation structures.
Its importance lies not only in the fact that it is a “standard” test, but also in the fact that it allows obtaining critical indicators for regulatory compliance: bending stiffness, yield strength, ultimate strength and deformation behavior. These results are essential to justify the mechanical performance of the product in the technical dossier and to reinforce the evidence required by MDR 2017/745.
Why it is used in medical devices
Implantable and structurally supported devices face a demanding environment: physiological loads, repeated movements, micro-impacts and conditions that can accelerate fatigue. A component failure due to bending can lead to loss of stability, pain, reoperation or device removal.
The four-point bend test helps answer key questions for R&D, quality and regulatory teams:
- Does the design withstand the expected loads without permanent deformation?
- Is the stiffness adequate to maintain function without compromising biomechanics?
- How does performance compare with previous versions or equivalent products?
- Are the results consistent and reproducible to support a regulatory assessment?
How the four-point bending test works
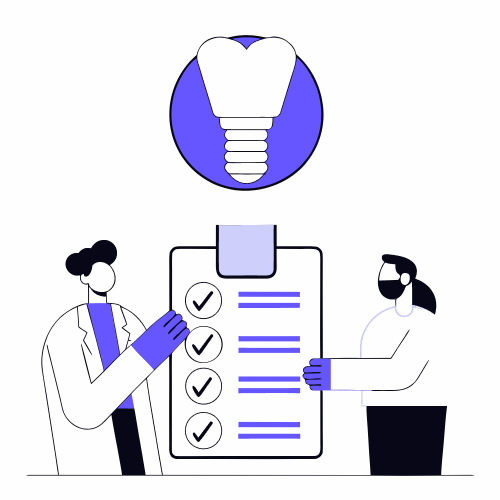
In simple terms, the test applies load to a specimen or component by means of four supports:
- Two external supports (support)
- Two inner supports (load application points)
Unlike three-point bending (a single point of central load), four-point bending generates a constant bending moment zone between the two interior points. This is valuable because:
- Reduces the concentration of stresses in a single point
- Evaluates the behavior of the material or component over a wider region
- Allows to identify more clearly the elastic and plastic response of the element.
In devices such as bone plates, this is especially useful to simulate how stresses are distributed when the implant works as a “bridge” between bone segments.
What is measured in a four-point bending test?
Typical results include metrics directly applicable to design and validation:
Bending stiffness (N/mm or equivalent)
Indicates how much the component deforms under a given load. It is key to check structural stability and functional behavior.
Maximum bending strength
Maximum load supported before failure or reaching a deformation threshold defined by the standard or protocol.
Elastic limit and permanent deformation
Helps to identify at what load level the component stops recovering its shape, which is critical in implants that must maintain alignment and stability.
Load-displacement curve
Allows comparison of designs, materials and manufacturing processes, and serves as technical evidence in the regulatory dossier.
Typical applications in the health sector
The four-point bending test is frequently used in:
Osteosynthesis and bone fixation plates
It is one of the most representative tests to validate plates used in traumatology and maxillofacial, in combination with recognized standards such as ISO 9585 and ASTM F382 (according to plate geometry and typology).
Implantable metal components
Structures working under bending loads, such as certain instrumentation parts or support components, can be validated with this method to establish their mechanical behavior under load.
Support devices and technical aids
Certain non-implantable products with strength requirements (e.g. support structures) may employ four-point bending as a robust and repeatable test.
Relationship to MDR 2017/745 and evidence of compliance.
The MDR does not impose a closed list of tests, but it does require the manufacturer to demonstrate, with verifiable evidence, that the product meets general safety and performance requirements. For devices working under loads, mechanical evidence is a central part.
Therefore, four-point bending tests are usually integrated within:
- Preclinical validation of performance
- Design rationale and material selection
- Comparisons with equivalent products
- Risk analysis and verification of mitigations
- Technical dossier for Notified Body assessment
When the test is used as evidence for CE marking, it is common for it to be performed in an ISO/IEC 17025 accredited laboratory, as it improves the acceptability of the report and reduces the risk of observations or retesting.
Best practices for reliable results
A well-planned assay is not just “putting a load on” and recording a value. To ensure useful and defensible results, it is recommended:
- Clearly define geometry, mounting and support conditions
- Check tolerances and alignment to avoid artifacts
- Establish test speed and environmental conditions
- Verify equipment calibration and metrological traceability
- Determine failure criteria (fracture, deformation, normative limit)
- Document the entire process with photographs, diagrams and recorded curves.
In addition, when the component is a real implant (not an ideal specimen), it is important to adapt the setup to represent the clinical condition in a reasonable and reproducible way.
A fundamental process
The four-point bending test is critical because it allows the stiffness and flexural strength of structurally loaded devices to be assessed with high reliability, providing critical data for design, quality control and regulatory compliance. In the context of MDR, robust, traceable and comparable results are a decisive factor in accelerating validation and minimizing certification risks.
If you are developing osteosynthesis plates, implantable components or medical structures with mechanical demands, this test is often one of the baseline tests to demonstrate performance and safety.